Background: Hypomethylating agents (HMA), such as decitabine, azacitidine, and oral decitabine/cedazuridine, are standard-of-care therapies for patients with myelodysplastic syndromes (MDS). Though they improve peripheral blood cytopenia and delay disease progression, most patients eventually lose response to these agents, a phenomenon known as HMA failure (HMA-F). Survival outcomes are poor with no Federal Drug Administration-approved treatment options in the HMA-F setting. Here, we assess MDS patients treated with HMA for potential clinical and pathological predictors of HMA-F.
Methods: We retrospectively evaluated all untreated patients with MDS seen at a single tertiary cancer center between July 2017 and July 2021 and identified those who received HMA therapy after diagnosis. Baseline patient characteristics and bone marrow (BM) data, including morphology, cytogenetics, and mutations, were collected. Genomic DNA was extracted from whole BM aspirate samples and subject to 81-gene target PCR-based sequencing using a next-generation sequencing platform. Survival data was updated in July 2023.
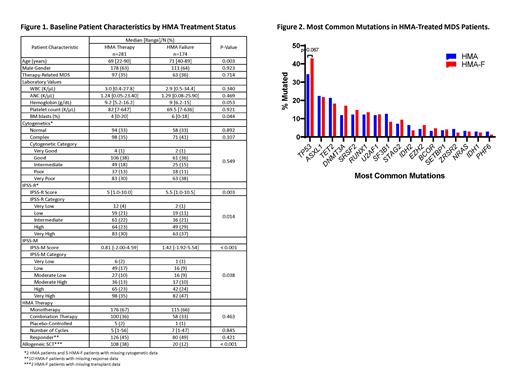
Results: A total of 799 untreated MDS patients were identified, and 455 patients (57%) eventually underwent treatment with HMA. With a median follow-up time of 46.8 months (95% confidence interval (CI): 44.4, 52.4), 174 patients (38%) were refractory to or later progressed on HMA therapy. Figure 1 depicts the differences in baseline clinical characteristics in those who remained on HMA therapy and those who developed HMA-F. HMA-treated patients who eventually developed HMA-F were more likely to be older (p=0.003), have higher BM blasts (p=0.044), and higher-risk by both IPSS-R (p=0.003) and IPSS-M (p<0.001) scoring systems. No differences were observed by HMA treatment type, number of cycles, and response. More patients underwent allogeneic stem cell transplantation while on frontline HMA therapy than after development of HMA-F (p<0.001).
Figure 2 shows the distribution of mutations detected in at least 10 patients between individuals who remained on HMA therapy and those who developed HMA-F. TP53 mutations tended to be more prevalent in those who developed HMA-F (p=0.067). No mutations were detected in 31 patients (11%) on HMA therapy and 13 patients (8%) with HMA-F (p=0.222). Interestingly, patients who remained on HMA therapy more frequently had multiple mutations in the same gene than those who developed HMA-F (58 patients (21%) vs 12 patients (7%), p<0.001).
The median overall survival from MDS diagnosis was 35.9 months (95% CI: 31.9, 55.3) in those who remained on HMA therapy vs 22.5 months (95% CI: 18.9, 25.7) in those who developed HMA-F (p<0.001). When only accounting for patients who underwent SCT, there was no difference in survival between the two groups (current HMA therapy 53.9 months vs HMA-F 58.1 months, p=0.541). In the HMA-F cohort, the median overall survival from the time of HMA-F was 6.8 months (95% CI: 5.5, 9.6).
Conclusions: In this group of MDS patients treated with HMA, patients who eventually developed HMA-F were older with higher BM blasts and higher-risk disease by IPSS-R and IPSS-M. No statistically significant differences were observed by HMA therapy details, cytogenetics, and specific mutations, but those with multiple mutations in the same gene tended to remain on HMA therapy. Further investigation into predictors of HMA-F and potential other treatment modalities is warranted.
Disclosures
Chien:Rigel Pharmaceuticals: Consultancy; AbbVie: Consultancy. Montalban-Bravo:Rigel: Research Funding; Takeda: Research Funding. Short:AstraZeneca: Consultancy; Astellas: Research Funding; Amgen: Honoraria; Stemline therapeutics: Research Funding; Takeda: Consultancy, Research Funding; Novartis: Consultancy; Pfizer: Consultancy. Jabbour:Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Adaptive Biotech: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Ascentage Pharma Group: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Hikma Pharmaceuticals: Consultancy, Honoraria, Research Funding. Kadia:Amgen, Inc.: Research Funding; Regeneron Pharmaceuticals: Research Funding; Sanofi-Aventis: Consultancy; SELLAS Life Sciences Group: Research Funding; Pulmotect, Inc.: Consultancy, Research Funding; Novartis: Consultancy; Genzyme: Honoraria; AstraZeneca: Research Funding; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; Celgene: Research Funding; Genentech: Consultancy, Research Funding; GenFleet Therapeutics: Research Funding; Hikma Pharmaceuticals: Speakers Bureau; Glycomimetics: Research Funding; Liberum: Consultancy; Cellenkos Inc.: Research Funding; Delta-Fly Pharma, Inc.: Research Funding; Ascentage Pharma Group: Research Funding; Cure: Speakers Bureau; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; Astellas Pharma Global Development: Research Funding; Pfizer: Consultancy, Research Funding; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; Janssen Research and Development: Research Funding; Cyclacel: Research Funding; Iterion: Research Funding; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; Agios: Consultancy; Servier: Consultancy; Pinotb-Bio: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria. Ravandi:Syros: Consultancy, Honoraria, Research Funding; Xencor: Research Funding; Biomea fusion: Honoraria, Research Funding; Prelude: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Astex/taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Honoraria, Research Funding. Borthakur:Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Pacylex, Novartis, Cytomx, Bio Ascend:: Membership on an entity's Board of Directors or advisory committees; Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy. Kantarjian:Amgen (Inst): Research Funding; Ascentage Pharma (Inst): Research Funding; Bristol-Myers Squibb (Inst): Research Funding; Amgen: Honoraria; Novartis (Inst): Research Funding; Ascentage Pharma Group: Honoraria; Astellas Pharma: Honoraria; AstraZeneca/MedImmune: Honoraria; Daiichih-Sankyo (Inst): Honoraria, Research Funding; Immunogen (Inst): Honoraria, Research Funding; Ipsen: Honoraria; Jazz Pharmaceuticals (Inst): Honoraria, Research Funding; KAHR Medical: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Precision Biosciences: Honoraria; Shenzhen Target Rx: Honoraria; Taiho Pharmaceutical: Honoraria; Abbvie (Inst): Research Funding; Abbvie: Consultancy, Honoraria. Garcia-Manero:Bristol Myers Squibb: Other: Medical writing support, Research Funding; Genentech: Research Funding; AbbVie: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal